This page shall be updated regularly upon discovery of research that fits the following parameters: research relating to the connection between valproate and autism.
Original Ground-Breaking Discovery by Autism Librarian in December 2024:
Since 2011 the FDA updated the package inserts for Valproate products to include risk of autism due to prenatal valproate exposure and omitted this detail from drug safety communications
In 2011 the FDA conducted a Drug and Safety Communication and announced that valproate was causing lower IQ in the offspring of mothers who took it during pregnancy:
[6-30-2011] The U.S. Food and Drug Administration (FDA) is informing the public that children born to mothers who take the anti-seizure medication valproate sodium or related products (valproic acid and divalproex sodium) during pregnancy have an increased risk of lower cognitive test scores than children exposed to other anti-seizure medications during pregnancy.
A similar announcement came in 2013:
[05-06-2013] The U.S. Food and Drug Administration (FDA) is advising health care professionals and women that the anti-seizure medication valproate sodium and related products, valproic acid and divalproex sodium, are contraindicated and should not be taken by pregnant women for the prevention of migraine headaches. Based on information from a recent study, there is evidence that these medications can cause decreased IQ scores in children whose mothers took them while pregnant.
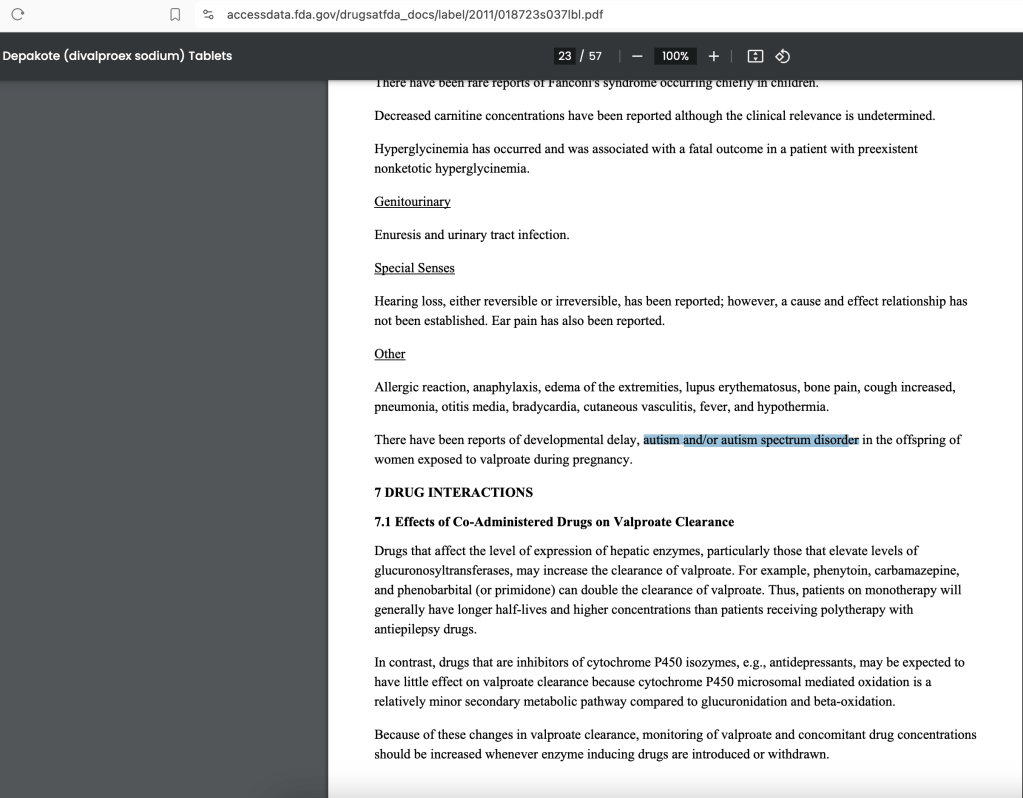
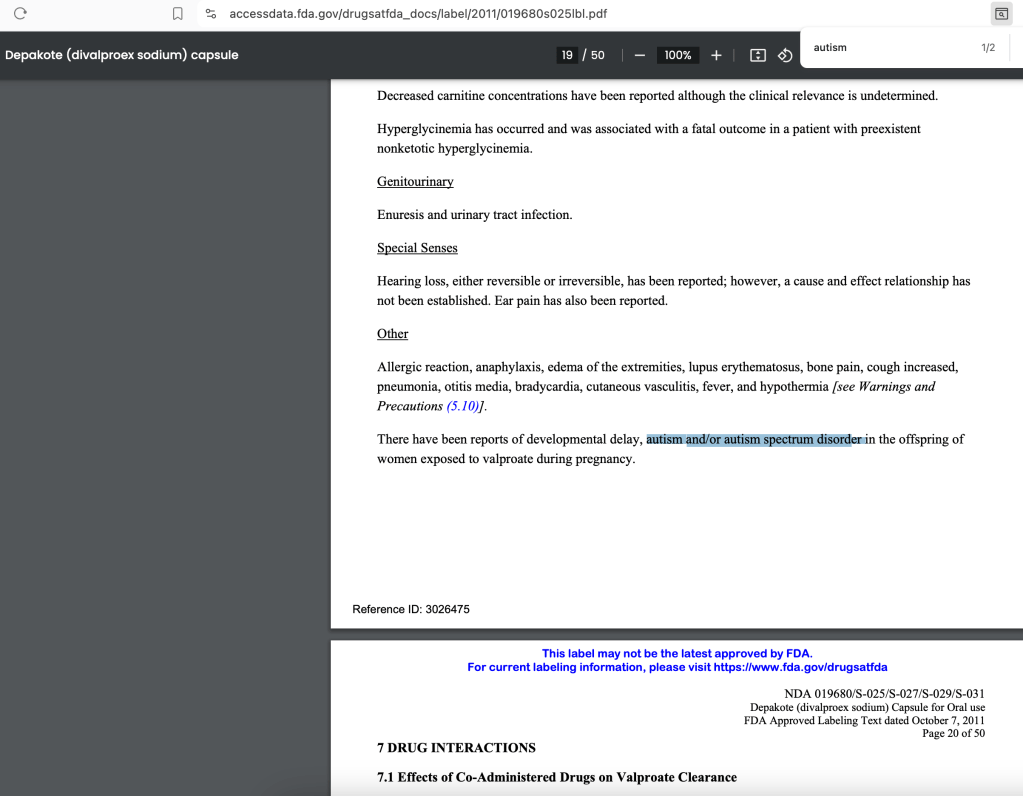
The FDA DID NOT NOTIFY THE PUBLIC to specify that it was AUTISM that was updated to the FDA package inserts for Valproate products.
Valproate products include: valproate sodium (Depacon), divalproex sodium (Depakote, Depakote CP, and Depakote ER), valproic acid (Depakene and Stavzor), and their generics.
Depacon Injection
Depacon was discontinued from the market. The FDA states “Federal Register determination that product was not discontinued or withdrawn for safety or effectiveness reasons.”



Depakote Delayed Release -Tablets


Depakote Delayed Release -Capsules


Depakote Extended Release Tablets
The latest package inserts are not available on the FDA website. The latest package insert can be found on the manufacturers site.


Depakote Delayed Release- Sprinkle Capsules




Depakene
Depakene was discontinued from the market in 2020. The FDA states “Federal Register determination that product was not discontinued or withdrawn for safety or effectiveness reasons.”



STAVZOR Delayed Release Capsules
Stavzor was discontinued from the market. The FDA states “Federal Register determination that product was not discontinued or withdrawn for safety or effectiveness reasons.”



June 2024 Discovery: Depakote online website lists ‘autism’ as a consequence for offspring of pregnant mothers exposed to the drug during pregnancy.

Press Release: Abbott Labs to Pay $1.5 Billion to Resolve Criminal & Civil Investigations of Off-label Promotion of Depakote Monday, May 7, 2012
“Global Health Care Company Abbott Laboratories Inc. has pleaded guilty and agreed to pay $1.5 billion to resolve its criminal and civil liability arising from the company’s unlawful promotion of the prescription drug Depakote for uses not approved as safe and effective by the Food and Drug Administration (FDA), the Justice Department announced today. The resolution – the second largest payment by a drug company – includes a criminal fine and forfeiture totaling $700 million and civil settlements with the federal government and the states totaling $800 million. Abbott also will be subject to court-supervised probation and reporting obligations for Abbott’s CEO and Board of Directors.”
“The civil settlement addresses broader allegations by the United States that from 1998 through 2008, Abbott unlawfully promoted Depakote for unapproved uses, including behavioral disturbances in dementia patients, psychiatric conditions in children and adolescents, schizophrenia, depression, anxiety, conduct disorders, obsessive-compulsive disorder, post-traumatic stress disorder, alcohol and drug withdrawal, attention deficit disorder and autism.”
Smolinski, N. E., Sarayani, A., Thai, T. N., Jugl, S., Ewig, C. L. Y., & Winterstein, A. G. (2024). Prenatal Exposure to Valproic Acid Across Various Indications for Use. JAMA network open, 7(5), e2412680. https://doi.org/10.1001/jamanetworkopen.2024.12680
- This study examined a 15 year time span across January 1, 2005, to December 31, 2020 and found that Valproic Acid use during pregnancy did not decline in spite of warnings by the FDA.
- Prescription epilepsy declined, while prescription for mood and migraines doubled during the study period.
- Only 22.3% of treatment with Valproic Acid had a 1-day overlap with contraception use.
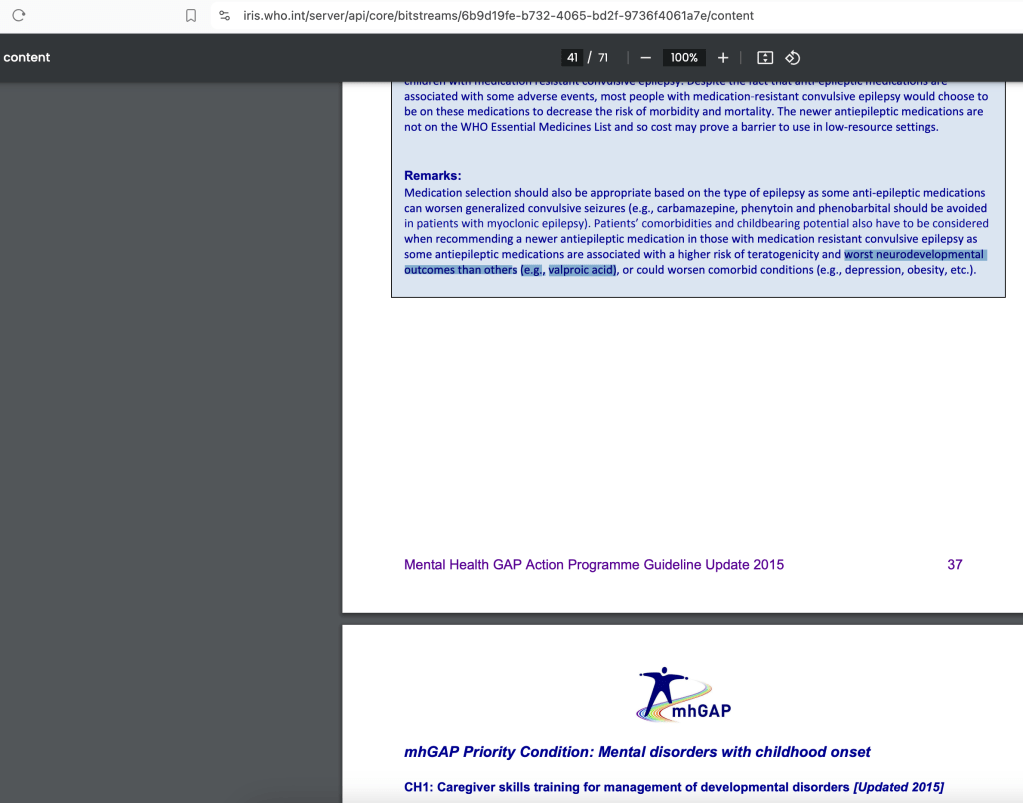
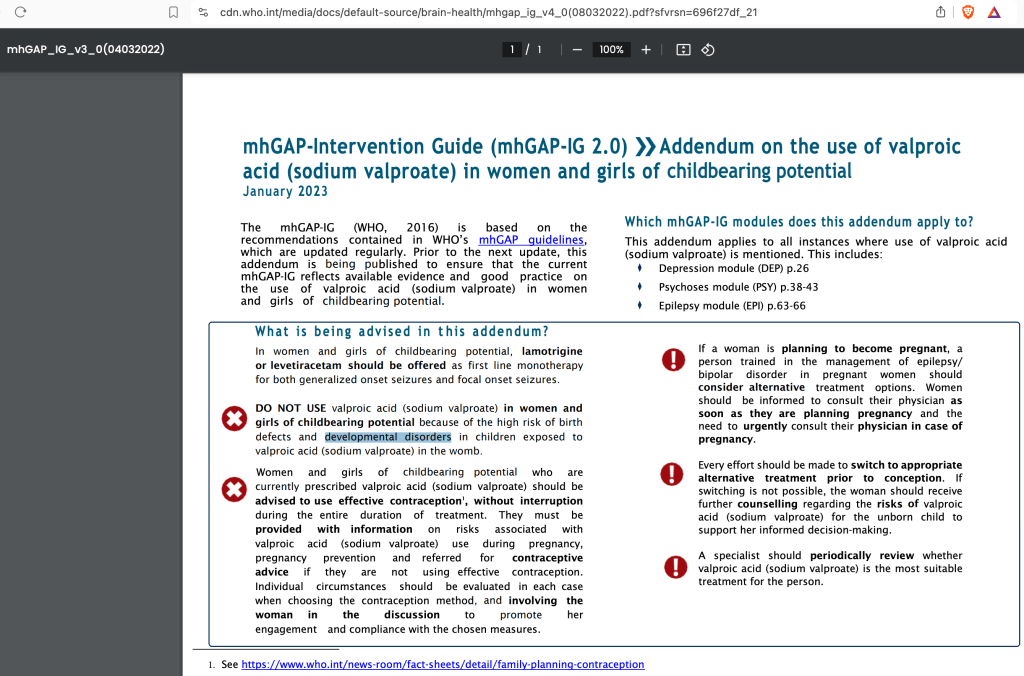
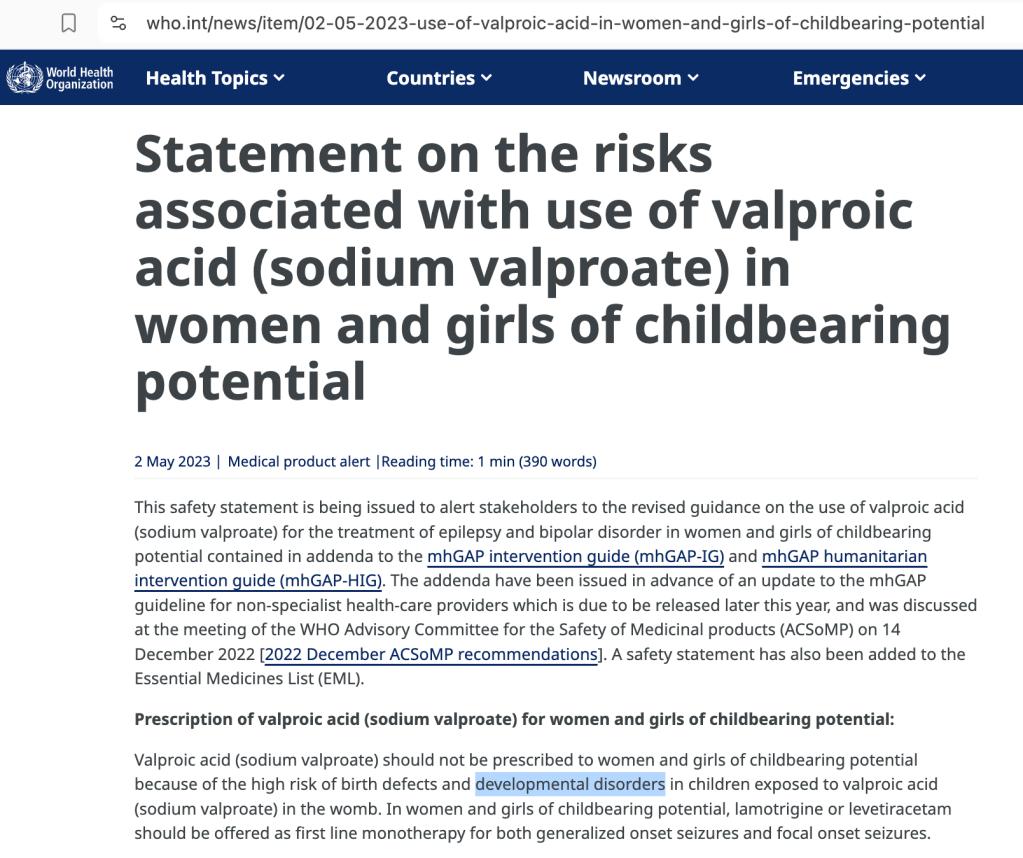
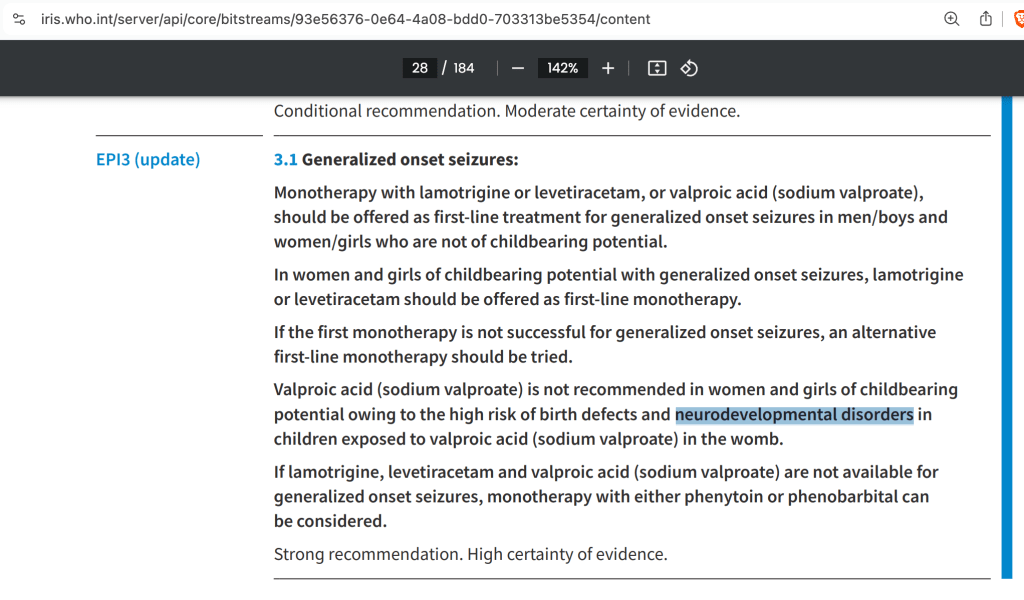
Is the World Health Organization Aware of the Valproate-Autism connection?
On June 16, 2025 I published Trump’s Executive Order: Is Exiting the WHO the Right Thing to Do (for Autism)? highlighting several key documents where the WHO specifically warns against valproate use by woman of childbearing potential due to developmental effects on children.