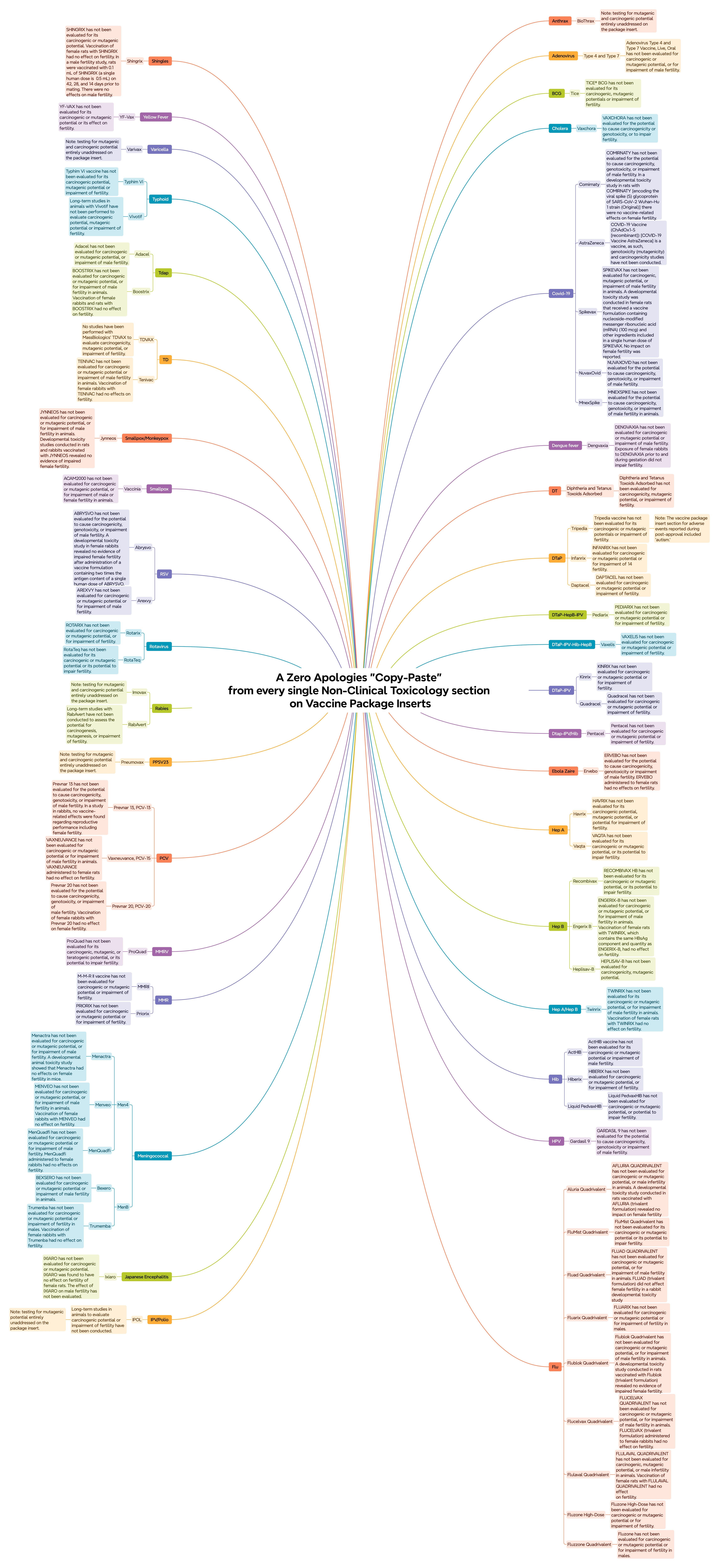
Review: After review of all package inserts for vaccines, in January of 2025 I announced my analysis of a section titled ‘Non-Clinical Toxicology’ as it concerned implications not previously considered. For all vaccines the ‘Non-Clinical Toxicology’ section stated the vaccine had never undergone testing for mutagenic or carcinogenic potential safety tests.
Until recently, Non-Clinical Toxicology has not been a part of vaccine discourse.
A few package inserts omit the section entirely, by omission also declaring lack of mutagenic and carcinogenic potential safety tests. Were someone to make the claim that the vaccine did undergo those tests to support the claim that the vaccines are ‘safe,’ they can be asked for that data.
Genotoxicity and the US Department of Health and Human Services
Since as early as 1996, the US Department of Health and Human Services has provided industry guidance on genotoxicity testing of pharmaceuticals [1].
Since as early as 1997, the US Department of Health and Human Services has known that, “It is clear that no single test is capable of detecting all relevant genotoxic agents.” [2]
If no single test is capable of detecting all genotoxic agents and this was known by HHS since as early as 1997, how is it that the Department of HHS has never filed a report to Congress on how they have made vaccines ‘safer’ within the glaring context of Non-Clinical Toxicology which declares on the vaccine package inserts themselves that the vaccine has never been evaluated for mutagenic/genotoxic potential?
The Department of HHS can provide industry guidelines for genotoxicity testing of other pharmaceuticals, but not improve the safety of vaccines within the realm of Non-Clinical Toxicology?
- “It is appropriate to assess genotoxicity in a bacterial reverse mutation test.” [2]
- “DNA damage considered to be relevant for mammalian cells and not adequately measured in bacteria should be evaluated in mammalian cells.” [2]
- “An in vivo test for genetic damage should usually be a part of the test battery to provide a test model in which additional relevant factors (absorption, distribution, metabolism, excretion) that may influence the genotoxic activity of a compound are included.” [2]
- “The following standard test battery is recommended based upon the considerations mentioned above:
- (i) A test for gene mutation in bacteria.
- (ii) An in vitro test with cytogenetic evaluation of chromosomal damage with mammalian cells or an in vitro mouse lymphoma tk assay.
- (iii) An in vivo test for chromosomal damage using rodent hematopoietic cells.” [2]
No vaccine has undergone the standard 3-test battery as recommended by the Department of Health and Human Services for pharmaceuticals in 1997; as the “vaccine schedule” stands, there are cumulative vaccine doses across a lifespan as well as multiple doses in a single doctor’s visit to also consider in this regard. In other words, the 3-test battery recommended in 1997 by HHS may be conducted for a single vaccine; but those results will not have external validity to real-world scenarios of cumulative doses across a lifespan, or multiple dose administrations in a single visit to the doctor.
- “It is also apparent that excessive toxicity often does not allow a proper evaluation of the relevant genetic endpoint. [1]
- Indeed, at very low survival levels in mammalian cells, mechanisms other than direct genotoxicity per se can lead to “positive” results that are related to cytotoxicity and not genotoxicity (e.g., events associated with apoptosis, endonuclease release from lysosomes, etc.). Such events are likely to occur once a certain concentration threshold is reached for a toxic compound.” [1]
Were vaccines to undergo a battery of tests and found to have such excessive toxicity that it doesn’t allow evaluation of a genetic endpoint due low survival levels in mammalian cells via cytotoxicity, can vaccines continue to be marketed as ‘safe’? Is this the reason vaccines have not undergone mutagenic and carcinogenic potential safety tests, due to excessive toxicity that renders it difficult to even study genetic mutations or carcinogenic potential?
The health agencies will have to justify why Non-Clinical Toxicology safety tests have not been conducted for vaccines. Negligence? Excessive toxicity of vaccine contents that make it difficult to study mutagenic and carcinogenic potential? Neither of these would be proper justification; especially the latter, as an “excessive toxicity” defence will only anger the public and make some recipients feel yet further that they are being experimented on with vaccines that have not undergone these safety tests prior to market release.
The latest ‘Guidance for Industry’ publication in 2023 states,
“Assessment of the mutagenic potential of impurities as described in this guidance is not intended for the following types of drug substances and drug products: biological/biotechnological, peptide, oligonucleotide, radiopharmaceutical, fermentation products, herbal products, and crude products of animal or plant origin.” [3]
A vaccine is considered a biological product; and this is stated in an alternative FDA document that defines biological products. This could explain why there have not been mutagenic and carcinogenic potential safety tests for vaccines.
However, this does not ethically justify the lack of mutagenic and carcinogenic potential safety tests of vaccines within the greater context of vaccine injuries, vaccinated versus unvaccinated studies, the lack of scientific support for various marketing claims regarding vaccines, the NIH’s inability to provide scientific evidence for the safety of aluminum adjuvants, etc. Additionally the establishment of the National Childhood Vaccine Injury act for ‘injury or death’ arising out of vaccines, the establishment of the National Vaccine Injury Compensation system, the establishment of the National Vaccine Advisory Committee, would only add weight for the necessity to conduct mutagenic and carcinogenic potential safety tests considering it has been known since 1986 and earlier that vaccines may result in “injury or death.”
Most of the public is unaware the term “injury or death” is explicitly stated as a potential consequence of vaccines in the National Childhood Vaccine Injury Act of 1986 -and those two words ‘injury’ and ‘death’ are found on that document dozens of times; why it even includes a vaccine injury table.
This year will mark 40 years since the National Childhood Vaccine Injury Act of 1986.
Forty years later,
countless injuries forty years later,
countless deaths forty years later,
there have been no mutagenic and carcinogenic potential safety tests to address the question: why are these happening in the first place?
Unfortunately, we don’t have these answers regarding vaccines; as the Non-Clinical Toxicology section for every single vaccine indicates it was never tested for mutagenic or carcinogenic potential.
Thus, let us examine 9 ways we can hold constructive, non-berating conversations about this neglected section on vaccine package inserts and how this section changes vaccine discourse moving forward:
- Location of Evidence
- The evidence is on the vaccine package inserts themselves.
The package inserts for vaccines can be found online through various sources and are publicly available through the efforts of various organizations that had to scour the internet looking for the package inserts, as these, as they should be, are not easily accessible on the CDC, NIH, FDA’s website in spite of mass vaccination mandates.
No one can argue it’s “unscientific” or a “conspiracy theory” to say “no vaccine has undergone such tests for mutagenic or carcinogenic potential,” as the evidence is on the package inserts themselves.
Anyone can look up and be shown a PDF file of a vaccine package insert, you can search ‘non-clinical toxicology,’ screenshot that section, and share it with friends.
The evidence is at your fingertips.
People cannot dispute the evidence on the vaccine package insert; it was created by the vaccine manufacturer and approved by the FDA.
Dare you contradict the vaccine package insert that was approved by the FDA?!
- The evidence is on the vaccine package inserts themselves.
- “Vaccines Save Lives” is a Separate Discussion Entirely
- Non-Clinical Toxicology is a discussion outside all other present scientific publications.
To my knowledge, there are no published scientific studies to address the following questions:
1. Even if a vaccine does protect against a disease it’s purported to protect, what if via genetic and carcinogenic potential the risk of other diseases IN ONESELF is increased by vaccine administration of single or cumulative doses across the lifespan?
2. Even if a vaccine does protect against a disease it’s purported to protect, what if via genetic and carcinogenic potential the risk of other diseases IN THE OFFSPRING is increased by vaccine administration of single or cumulative doses across the lifespan?”
- Non-Clinical Toxicology is a discussion outside all other present scientific publications.
- Marketing: Can any vaccine be marketed as ‘safe’ within the context of Non-Clinical Toxicology?
- Vaccines are marketed as ‘safe and effective,’ yet can such “safe” marketing remain under the context of Non-Clinical Toxicology?
Can any vaccine be marketed as ‘safe’ for the recipient if the health-agency/vaccine-manufacturer cannot scientifically defend the claim that a vaccine does not mutate genes?
Can any vaccine be marketed as ‘safe’ for the recipient if the health-agency/vaccine-manufacturer cannot scientifically defend the claim that a vaccine does not increase the risk of cancers?
Can any vaccine be marketed as ‘safe’ to the recipient’s future offspring if the health-agency/vaccine-manufacturer cannot scientifically defend the claim that a vaccine does not mutate genes in parents whose mutations may be passed onto offspring?
Can any vaccine be marketed as ‘safe’ to the recipient’s future offspring if the health-agency/vaccine-manufacturer cannot scientifically defend the claim that a vaccine does not increase the risk of cancers in the offspring?
Does marketing a vaccine as ‘safe’ due to protection against one disease but possibly not another due to possible genetic/carcinogenic potential, make a vaccine manufacturer liable for fraudulent marketing of the vaccine as being ‘safe’ due to failure to warn of the lack of mutagenic and carcinogenic potential safety tests? Should vaccines carry a safety label indicating “it is unknown whether this product may increase the risk of other diseases or cancer due to lack of mutagenic and potential safety tests”?
- Vaccines are marketed as ‘safe and effective,’ yet can such “safe” marketing remain under the context of Non-Clinical Toxicology?
- Informed consent
- Ironically, there would be no need for informed consent in the medical marketplace if drugs and vaccines did not have side effects/adverse events to begin with.
The very notion of informed consent is based upon the premise that a consumer needs to be informed regarding the potential harms of a medical treatment in order to make an “informed decision,” oftentimes under the pressure of a social context (e.g., hospital, doctors, nurses, not being provided alternative healthcare options in that setting, lack of education or awareness of alternative healthcare options, etc.).
In other words, informed consent is necessary because potential harm from a product is already known.
Within the context of Non-Clinical Toxicology, informed consent regarding mutagenic and carcinogenic potential would require providing the consumer a statement such as, “while scientific evidence supports the assertion that this vaccine may protect you against a disease, there are no studies that determine this product is without mutagenic or carcinogenic potential risk. Taking this product may or may not increase the risk of other diseases and cancers.”
Wouldn’t that be ethical to do?
Has lack of informing consumers regarding Non-Clinical Toxicology been unethical within the context of informed consent provision to vaccine recipients?
- Ironically, there would be no need for informed consent in the medical marketplace if drugs and vaccines did not have side effects/adverse events to begin with.
- Do vaccines mutate genes that may or may not cause autism?
- In the absence of assays to determine mutagenic and carcinogenic potential of vaccines, there is no answer to this question within the realm of Non-Clinical Toxicology as every single package insert states those safety tests were never done.
Autism is considered a heterogeneous disorder; meaning, it may differ greatly from person to person. Could parental and offspring genetic mutations from vaccine administration explain the differences in autism spectrum disorder presentation (aka the heterogeneity of autism) across individuals?
Sadly, the public at large believes autism to be a highly heritable, genetic disorder. How can they continue to believe this when Non-Clinical Toxicology becomes more widely known?
As it stands, no government health official can make the claim ‘vaccines do not mutate genes that lead to autism’ with scientific support for the claim.
Can the statement ‘vaccines do not cause autism’ continue to be stated by government health agencies within the context of Non-Clinical Toxicology?
- In the absence of assays to determine mutagenic and carcinogenic potential of vaccines, there is no answer to this question within the realm of Non-Clinical Toxicology as every single package insert states those safety tests were never done.
- Other Genetics Literatures
- In the broadest sense concerning all products on the market approved by the FDA, can government health representatives make the statement ‘autism is hereditary disorder impacted by genes’ if even a single product they’ve approved retroactively and moving forward has never undergone mutagenic potential safety tests to rule out its impact on other diseases?
How do other genetics literatures stand within the realm of Non-Clinical Toxicology?
Arguably, if even a single person within a dataset of a genetics research study has been subjected to increased risk to a disease/cancer due to a product that never underwent Non-Clinical Toxicology safety tests, and the product may be the real culprit behind increase risk of a disease, the interpretation of the results of the genetics publication may be compromised if it does not address all the pharmaceutical products the dataset was exposed to -with and without mutagenic and carcinogenic potential safety tests.
What foundation do genetics literatures that do not account for pharmaceutical exposure within the realm of Non-Clinical Toxicology stand upon?
- In the broadest sense concerning all products on the market approved by the FDA, can government health representatives make the statement ‘autism is hereditary disorder impacted by genes’ if even a single product they’ve approved retroactively and moving forward has never undergone mutagenic potential safety tests to rule out its impact on other diseases?
- Do the benefits really outweigh the risks? …of unknown mutagenic and carcinogenic risks?
- It should be a personal choice, it should be part of informed consent, for an individual to decide if the benefits of protection against a disease is worth the unknown risks of genetic mutations or cancers.
Notwithstanding challenges to assertions that vaccines are responsible for reduction in mortality rates of various diseases already on decline prior to the introduction of vaccines, -addressed elsewhere in this library- if the person believes a vaccine can protect them against a disease because of lack of education regarding data that says otherwise, the individual should have the right to be provided a greater context, the Non-Clinical Toxicology context, regarding the potential risks.
- It should be a personal choice, it should be part of informed consent, for an individual to decide if the benefits of protection against a disease is worth the unknown risks of genetic mutations or cancers.
- Is it unethical to not vaccinate someone to conduct a vaccinated versus unvaccinated study, but is it ethical to vaccinate someone without Non-Clinical Toxicology tests to show a vaccine does not mutate genes?
- It has been argued by some individuals that it is unethical to do vaccinated versus unvaccinated studies because it is ethical to vaccinate a person against a disease rather than to leave them “exposed” or “at risk” for the disease.
However, within the context of Non-Clinical Toxicology, just how ethical is it to vaccinate a person to begin with in the absence of Non-Clinical Toxicology safety tests?
How ethical is it to NOT KNOW the genetic and carcinogenic potential risks of a single vaccine administration, multiple vaccine administration in a single visit, or cumulative vaccines across the lifespan of an individual?
How ethical is it to vaccinate a person without knowing these risks?
If someone argues it is unethical to not vaccinate a person, within the realm of Non-Clinical Toxicology, it can be also argued it is unethical to vaccinate a person without knowing mutagenic and carcinogenic potential risks that increase the risks of other diseases.
- It has been argued by some individuals that it is unethical to do vaccinated versus unvaccinated studies because it is ethical to vaccinate a person against a disease rather than to leave them “exposed” or “at risk” for the disease.
- Will we ever see mutagenic and carcinogenic potential safety tests for single doses, multiple doses in a single visit, or cumulative exposure across a lifespan?
- The American public should demand that research, plain and simple.
Vaccines have been marketed as ‘safe’ while the Non-Clinical Toxicology section of vaccine package inserts indicates the opposite of safety via declaration of never doing such safety tests. The Vaccine Adverse Event Reporting System exploded in submissions after the Covid-19 vaccines were put on market.
If one purpose of health agencies is to protect public health, how is public health being protected without such safety tests?
- The American public should demand that research, plain and simple.
Forty years later since the National Childhood Vaccine Injury Act of 1986,
countless injuries forty years later,
countless deaths forty years later,
there have been no mutagenic and carcinogenic potential safety tests to address the question: why are these happening in the first place?
References
- Guideline for Industry Specific Aspects of Regulatory Genotoxicity Tests for Pharmaceuticals. April 1996 ICH S2A
- This guideline was developed within the Expert Working Group (Safety) of the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) and has been subject to consultation by the regulatory parties, in accordance with the ICH process. This document has been endorsed by the ICH Steering Committee at Step 4 of the ICH process, July, 1995. At Step 4 of the process, the final draft is recommended for adoption to the regulatory bodies of the European Union, Japan and the USA. This guideline was published in the Federal Register on April 24, 1996 (61 FR 18199) and is applicable to drug and biological products. Although this guideline does not create or confer any rights for or on any person and does not operate to bind FDA or the industry, it does represent the agency’s current thinking on recommended methods for testing and assessing the genotoxic potential of pharmaceuticals.
- Guidance for Industry S2B Genotoxicity: A Standard Battery for Genotoxicity Testing of Pharmaceuticals. U.S. Department of Health and Human Services. Food and Drug Administration. Center for Drug Evaluation and Research (CDER). Center for Biologics Evaluation and Research (CBER). July 1997 ICH.
- M7(R2) Assessment and Control of DNA Reactive. (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk. Guidance for Industry. U.S. Department of Health and Human Services. Food and Drug Administration. Center for Drug Evaluation and Research (CDER). Center for Biologics Evaluation and Research (CBER). July 2023. ICH-Multidisciplinary.
- Foreword: The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has the mission of achieving greater regulatory harmonization
- worldwide to ensure that safe, effective, and high-quality medicines are developed, registered, and maintained in the most resource-efficient manner. By harmonizing the regulatory expectations in regions around the world, ICH guidelines have substantially reduced duplicative clinical studies, prevented unnecessary animal studies, standardized safety reporting and marketing application submissions, and contributed to many other improvements in the quality of global drug development and manufacturing and the products available to patients.
- ICH is a consensus-driven process that involves technical experts from regulatory authorities and industry parties in detailed technical and science-based harmonization work that results in the development of ICH guidelines. The commitment to consistent adoption of these consensus-based guidelines by regulators around the globe is critical to realizing the benefits of safe, effective, and high-quality medicines for patients as well as for industry. As a Founding Regulatory Member of ICH, the Food and Drug Administration (FDA) plays a major role in the development of each of the ICH guidelines, which FDA then adopts and issues as guidance to industry.