Tylenol/acetaminophen
This week President Trump announced that prenatal exposure to acetaminophen during pregnancy could be contributing to risk of autism.
This is certainly a step in the right direction in regards to toxins associated with autism. Having recently published the latest scientific evidence from the available literature on acetaminophen and autism, I laud the announcement.
Responses on social media to the announcement have varied, with some celebrating the announcement and others criticizing it. Unfortunately, some individuals have made statements about intentionally taking Tylenol during pregnancy, or that if acetaminophen was truly causing autism the US would be in much better form. Such statements, while they garner some support, are not grounded in scientific evidence and harm empirical inquiry.
The psychological state of affairs regarding autism is certainly a matter the field of psychology should study objectively given public response on this matter is not always based in scientific evidence. Arguably, much public response is the consequence of various social phenomena (e.g., news outlets’ improper literature reviews, interviews of unqualified individuals with social or political influence seeking further self-aggrandizement, the prioritization of social influence and funding over scientific evidence, the very competitive nature of social media that prioritizes any publicity versus the delivery of scientific objectivity, so-called “neurodiversity”, the social rewards of advocating for one argument over another, perceptions of loyalty to supporters due to having focused on one viewpoint over another creating biases toward supporters’ perspectives, etc.). The study of the political impact on public education on the scientific literature on toxins associated with autism will also be an area of study for the field of psychology in the future (e.g., conflicts of interest held by government officials).
Two days after the announcement, Vice President JD Vance seemingly backtracked on President Trump’s and Robert F. Kennedy Jr.’s statements on acetaminophen, ambiguously urging mothers to listen to their doctors, yet also saying he supports more research on the matter and that people should be concerned with side effects of medications.
It is presently unknown whether JD Vance has read the entirety of the autism-acetaminophen literature to make any public statement on the matter. The News Nation interviewer seemingly qualified JD Vance to address the matter by saying, “father of three, you’ve seen pregnancy up close, what should women take for pain?” While a parent can certainly provide data concerning their children (case reports are data that comprise case-control, cohort, and meta analyses studies), relying on case report anecdotal opinions from parents does not provide the public the full breadth of scientific inquiry on complicated datasets across an entire scientific literature.
Please review scientific evidence compiled in this library regarding acetaminophen and autism. It is my present hypothesis that if a factor associated with autism in human studies is also inducing autism-like traits in animal studies, where those animals have zero exposure to other toxins, then the toxin may be capable of inducing autism alone in humans prior to factoring co-exposure to other toxins. While more research on acetaminophen is certainly welcome for both prenatal and postnatal influence on risk of autism, the literature certainly points in that direction -and the present evidence for prenatal exposure is stronger than postnatal exposure.
Nevertheless, it can be stated that President Trump and Robert Kennedy Jr.’s warning on the risks of autism are well received by those versed in the acetaminophen literature. In light of recent reports on the internal emails obtained by Daily Caller indicating that acetaminophen manufacturers considered recommending against Tylenol during pregnancy based upon a 2018 review of the scientific literature [1], as well as the re-surfacing of a social media post by Tylenol in which they state they don’t recommend any of their products to be taken during pregnancy, the drive to educate the public on the potential risks of acetaminophen is only strengthened.
Vaccines
Announcements this week have also addressed vaccines, with Robert F. Kennedy Jr. once again assuring efforts are underway toward addressing the matter.
Regarding vaccines, several significant changes have occurred this year:
- Trump issued an executive order prohibiting federal funding to schools, colleges, and educational agencies that require students to receive a COVID-19 vaccination as a condition for attending
- Covid-19 vaccines are no longer recommended for pregnant women and children
- The CDC’s Advisory Committee on Immunization Practices (ACIP) now recommends chickenpox vaccine to be taken alone rather than in combination with the MMR vaccine, and the combination vaccine (MMR-V) is no longer recommended for children under 4 years
- HHS Adopted the ACIP recommendation to remove thimerosal from all U.S. flu vaccines
- HHS removed the 17 sitting members of the ACIP committee
- New ACIP members have already been announced
- Susan Monarez is no longer director of the CDC
- The FDA announced new Covid-19 vaccines will undergo placebo controlled clinical trials (not to be confused with vaccine-pseudo-placebos which are historically used in vaccine clinical trials rather than a saline-solution-placebo)
- The FDA has revoked Emergency Use Authorization for Covid-19 vaccines
These accomplishments are certainly additional steps in the right direction concerning factors associated with autism.
But is this enough?
In his speech Robert F. Kennedy Jr. says ‘no area is taboo’ in regards to addressing the factors associated with autism.
These words are surprising given that in January of this year I published in this library as well as on X an endorsement for Robert F. Kennedy Jr., tagging him and many others, announcing my discovery of the lack of mutagenic and carcinogenic potential safety tests for vaccines found in the Non-Clinical Toxicology section of vaccines (adding further weight to arguments regarding the lack of proper vaccine safety testing, and with additional implications regarding ‘informed consent’), and offered a few preliminary postulations regarding the discovery’s implications (i.e., can the CDC scientifically support the claim ‘vaccines do not cause autism’ for a disorder that is believed to have high genetic heritability if they’ve never evaluated vaccines for genetic toxicity?).
For some reason this discovery was omitted from this week’s announcements on strides the administration is taking on addressing autism.
An announcement using the following words could have provided informed consent to the public: the American public is informed that no vaccine has undergone mutagenic and carcinogenic potential safety tests, and this evidence is found on most vaccine package inserts publicly available online in a section titled Non-Clinical Toxicology for public inspection. This means your doctor cannot guarantee the vaccine cannot mutate genes or increase risk of cancers, as neither single dose administrations of vaccines nor cumulative doses of vaccines administrated across an entire lifespan has been studied by vaccine manufacturers for genotoxicity (the ability to increase the risk for various diseases) or the potential to increase the risk of cancers.
Informed consent has become an important topic in recent years and my hopes were that the American public would be provided yet greater informed consent in regards to vaccines through this discovery.
Unfortunately, most of the American public now continues to remain ignorant of the Non-Clinical Toxicology section of the vaccine package inserts, a novel discovery that tosses arguments regarding vaccine safety out of the window without well-designed studies that answer the question: are single and/or cumulative doses of vaccines resulting in genetic mutations or increased risk of various cancers? Vaccine manufacturers may not be eager to conduct such studies, as they might argue such studies may contribute to yet further vaccine hesitancy, an argument that removes informed consent from the power of the patient into the vaccine manufacturers themselves.
Time will tell how this discovery is managed by a public suffering the consequences of social media and propaganda.
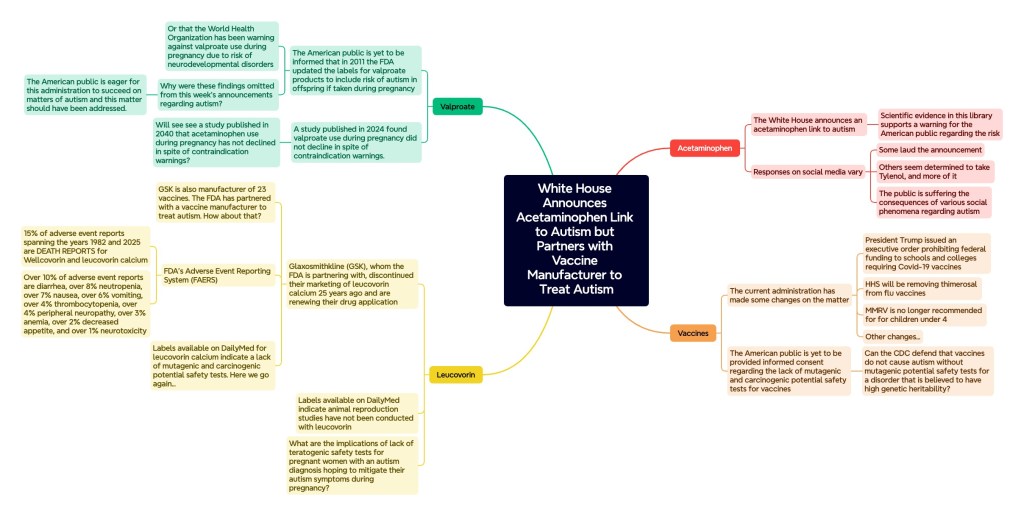
Valproate
HHS Secretary Kennedy speaks on the importance of addressing all factors associated with autism, yet omits warning the nation regarding valproate products.
In my aforementioned statement published on X and in this library, I once again announced that in 2011 the FDA updated package inserts for valproate products to include a risk of autism in the offspring if taken during pregnancy (the first announcement was in December of 2024). The FDA failed to mention this little detail in their 2011 and 2013 Drug Safety Communications. However, in spite of tagging Robert F. Kennedy Jr. and others on this important matter, the American public was not informed of this discovery this past week.
After President Trump pledged to withdraw from the World Health Organization, I conducted investigations into the WHO’s efforts surrounding autism and discovered that since as early as 2015 the WHO has been warning countries around the world against the use of valproate products during pregnancy due to neurodevelopmental concerns. However, the WHO has not specified the valproate-autism connection in these warnings. Are individuals at the World Health Organization aware of this link and omitting this particular detail from their publications?
While HHS Secretary Kennedy was quick to notify the public that physicians notices will be issued for acetaminophen, similar actions could have been taken for valproate products. While valproate products are already contraindicated during pregnancy, the American public should have been now notified regarding valproate’s link to autism. This is especially true given that a 2024 study published in JAMA examining a 15 year time span across January 1, 2005, to December 31, 2020 revealed that Valproic Acid use during pregnancy did not decline in spite of warnings by the FDA [2].
Will physicians and portions of the public also ignore warnings by the FDA regarding acetaminophen? Will we see a study published in 15 years that indicates acetaminophen use during pregnancy from 2025 through 2040 has not declined in spite of warnings by the FDA?
Time will tell how this discovery is managed by a public suffering the consequences of social media and propaganda.
Leucovorin
This weeks announcements by the White House also included a surprise: the FDA announced they have taken initial steps toward obtaining the approval of leucovorin calcium tablets for patients with cerebral folate deficiency (CFD), a deficiency often impacting those diagnosed with autism.

The FDA communication states, “CFD has also been reported in a broader patient population with neuropsychiatric symptoms, including autistic features, and detectable serum autoantibodies to the folate receptor alpha; however, there are limitations on the available data for the use of leucovorin in this population and additional studies are needed to assess safety and efficacy.”
Indeed, additional studies are desperately needed to assess safety and efficacy.
This is especially true provided previous package inserts for the oral tablet Wellcovorin (leucovorin calcium) by Glaxosmithkline (GSK), whom the FDA stated they are partnering with, are not available on the FDA website. Over 25 years ago, GSK notified the FDA they were no longer marketing the drug and on the Federal Register the FDA announced this week they are seeking a new drug application.
Glaxosmithkline, the infamous manufacturer of 23 different vaccines, has made headlines for various years.
In 2012, Glaxosmithkline was required to pay $3 billion due to fraud and failure to report safety data on various prescription drugs.
In 2014, Glaxosmithkline was fined $490 million by China due to the bribery of doctors to prescribe their drugs.
Most recently, in 2023 Glaxosmithkline was in the news due to concealing evidence showing their Zantac drug could cause cancer. Children’s Health Defense, a prominent organization in the vaccine safety advocacy movement founded by Robert F. Kennedy Jr., reported on the very matter and heavily criticized the vaccine manufacturer for its omission of safety data.
It is presently unknown why the Robert F. Kennedy Jr. is content with the FDA’s partnership with an organization facing over 70,000 Zantac lawsuits and a history of bribery and concealment of safety data.
Autism is a sensitive matter concerning various factors, with vaccines themselves undergoing ever-more scrutiny, and the FDA’s choice to partner with a vaccine manufacturer in the development for what is proposed to be an autism treatment is questionable.
Will GSK be allowed to have a financial hand in both a contributor to autism (vaccines) as well as the treatment for autism (leucovorin calcium)?
In regards to the efficacy of leucovorin and whether it is a viable treatment for autism, a meta analysis published in 2021 shows promising results [3].
However, has leucovorin been evaluated for mutagenic and carcinogenic potential in clinical trials?
Available labeling for leucovorin calcium tablets on DailyMed, an NIH website, indicate the drug has never undergone mutagenic and carcinogenic potential safety tests, a conclusion drawn since the Non-Clinical Toxicology section is entirely omitted from the labeling.
While the drug may show promising results in mitigating autism symptoms, what are the long-term effects of the drug? Can taking the drug increase the risk of certain cancers in spite of improving autism symptoms? Can the drug result in increased risk of other diseases in spite of improving autism symptoms?
In regards to teratogenic effects, all labels available on DailyMed for leucovorin tablets state the following:
“Animal reproduction studies have not been conducted with leucovorin. It is also not known whether leucovorin can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Leucovorin should be given to a pregnant woman only if clearly needed.”
The study of teratogenic effects is important given the increasing number of adults with an autism diagnosis who may look to leucovorin calcium to mitigate their own adult autism symptoms.
Will a vaccine manufacturer such as GSK withhold unfavorable results toward leucovorin treatment for autism should non-clinical toxicology or teratogenic potential tests reveal long-term health risks of the drug?
Will GSK even conduct non-clinical toxicology safety tests, rather than omitting them, as has been the case for every vaccine in history?
Will the USA see more non-clinical toxicology tests for greater numbers of drug products or vaccines, or will the US population continue to accept treatments for symptoms that could have short-term or long-term health risks?
The FDA recently launched their own Adverse Event Reporting System (FAERS) public dashboard, a database spanning many years of adverse event reports, and the results for leucovorin calcium and Wellcovorin (the brand previously marketed by GSK) speak for themselves.

Fifteen percent (15%) of adverse event reports spanning the years 1983 to 2025 are death cases.
Will parents wanting to improve autism symptoms in their children be provided this statistic as part of their informed consent?
Will adults with an autism diagnosis wanting to improve their own autism symptoms be provided this informed consent?
Overall, the number of adverse event reports increased exponentially from 1-2 reports per year in 1983-1984 to almost consistently a thousand reports per year since the year 2018, with the years 2022 and 2023 falling above 900 reports.
Will we continue to see an exponential increase in adverse event reports through the years now that the drug will be marketed as a treatment for autism symptoms?
Do the risks outweigh the benefits? Who gets to decide that?

Over 10% of adverse event reports are diarrhea, over 8% neutropenia, over 7% nausea, over 6% vomiting, over 4% thrombocytopenia, over 4% peripheral neuropathy, over 3% anemia, over 2% decreased appetite, and over 1% neurotoxicity.
Will parents and adults be provided informed consent regarding the potential side effects of the drug?
Time will tell how this announcement is managed by a public suffering the consequences of social media, propaganda, and Big Pharma giant GSK partnering with the FDA to treat autism.
References
- Bauer, A. Z., Kriebel, D., Herbert, M. R., Bornehag, C. G., & Swan, S. H. (2018). Prenatal paracetamol exposure and child neurodevelopment: A review. Hormones and behavior, 101, 125–147. https://doi.org/10.1016/j.yhbeh.2018.01.003
- Smolinski, N. E., Sarayani, A., Thai, T. N., Jugl, S., Ewig, C. L. Y., & Winterstein, A. G. (2024). Prenatal Exposure to Valproic Acid Across Various Indications for Use. JAMA network open, 7(5), e2412680. https://doi.org/10.1001/jamanetworkopen.2024.12680
- Rossignol, D. A., & Frye, R. E. (2021). Cerebral Folate Deficiency, Folate Receptor Alpha Autoantibodies and Leucovorin (Folinic Acid) Treatment in Autism Spectrum Disorders: A Systematic Review and Meta-Analysis. Journal of personalized medicine, 11(11), 1141. https://doi.org/10.3390/jpm11111141